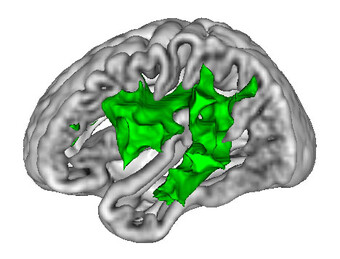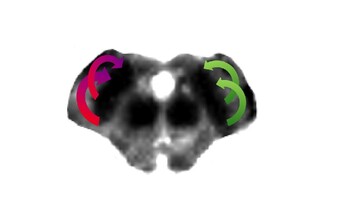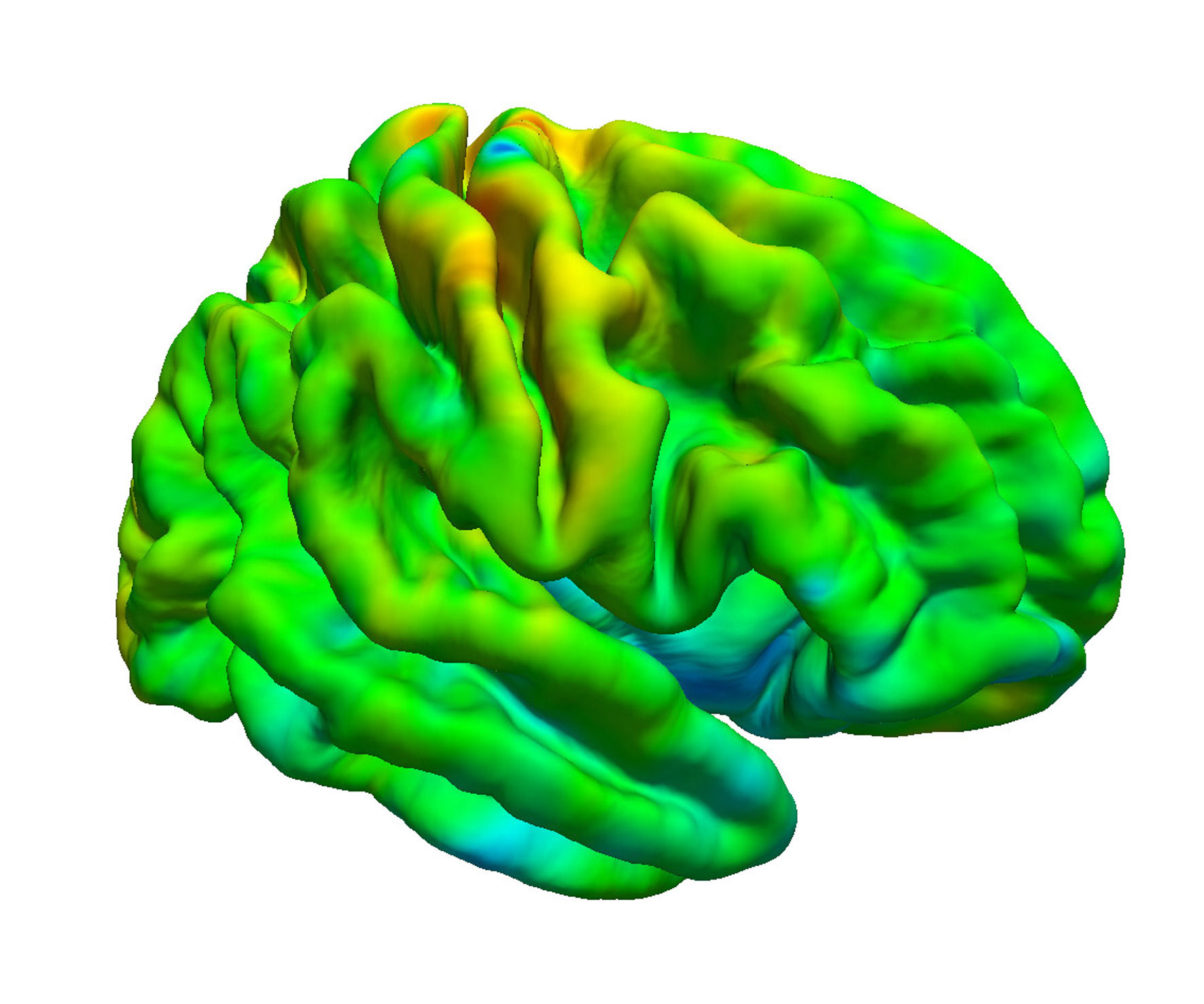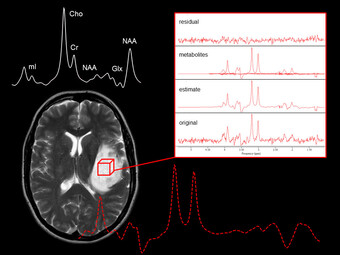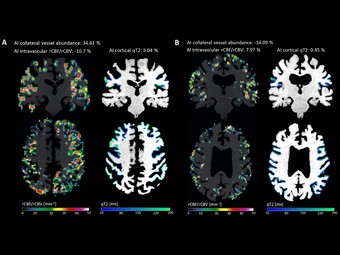
Regarding cerebrovascular diseases, one of our main research interests is investigating pathological changes of brain energy metabolism and cerebral microstructure in patients with chronic cerebral hypoperfusion. Due to the prognostic significance of altered brain oxygen metabolism concerning the occurrence of permanent ischemic tissue damage and the risk of (recurrent) ischemic stroke, the MRI-based characterization of tissue oxygen metabolism in patients with cerebral large-artery steno-occlusive disease has been one of our major research activities in recent years (Seiler et al., 2012, Stroke, Seiler et al., 2016, PLOS ONE, Seiler et al., 2019, Stroke), which we are further pursuing for continuous improvement and validation of oxygenation-sensitive MR imaging techniques. Furthermore, were are interested in hypoperfusion-associated tissue damage on the microstructural level and its potential link to cognitive impairment (Seiler et al., 2018, Am J Neuroradiol, Seiler et al., 2020, J Cereb Blood Flow Metab). Apart from the investigation of chronic hemodynamic impairment, part of our research focuses on methodological approaches for the assessment of collateral supply (Seiler et al., 2019, J Cereb Blood Flow Metab) and the metabolic description of tissue-at-risk in acute ischemic stroke (Seiler et al., 2017, Stroke, Seiler et al., 2019, Clin Neuroradiol, Seiler et al., 2019, J Cereb Blood Flow Metab). Further projects involve the evaluation of potential imaging biomarkers of cognitive decline in patients with small vessel disease as well as lesional and perilesional tissue characterization in patients with intracerebral hemorrhage.
Cooperations: -Prof. R. Deichmann, Head of the core structure of the BIC
-PD S. Baudrexel, Movement Disorders Group
-PD J. Klein, University of Oxford
-Prof. E. Hattingen, Institute of Neuroradiology
-Prof. M. Wagner, Betanien hospital
-Prof. C. Förch, Multiple Sclerosis Group
-Prof. F. Rosenow, Epilepsy Center Frankfurt
-Prof. S. Knake, Epilepsy Center Marburg
Members
Publications