Mathematical modeling to explore the role of Ub-receptors in the clearance of intracellular pathogens
Autophagy is a fundamental defense mechanism against invading pathogens. Pathogens, like Salmonella, that invade the host cytosol are targeted with ubiquitin for the autophagic pathway. It is very important to understand this process to find new therapies for invading pathogens, which more and more frequently become resistant to antibiotics.
To gain a better understanding of the biological system of antibacterial autophagy, we use systems biology approaches. The goal of the project is to develop a first mathematical model of ubiquitin dependent host-pathogen interactions. We apply the Petri net formalism to model the processes of Salmonella ubiquitination and the recognition of the autophagy receptors. Beside this, we are interested in the influence of inflammation pathways and the NF-?B pathway induced by Salmonella infection to compare the processes of the LPS-induced inflammation and the sterile inflammation. We apply the tool MonaLisa for the construction, the analysis, and the simulation of the Petri net model.
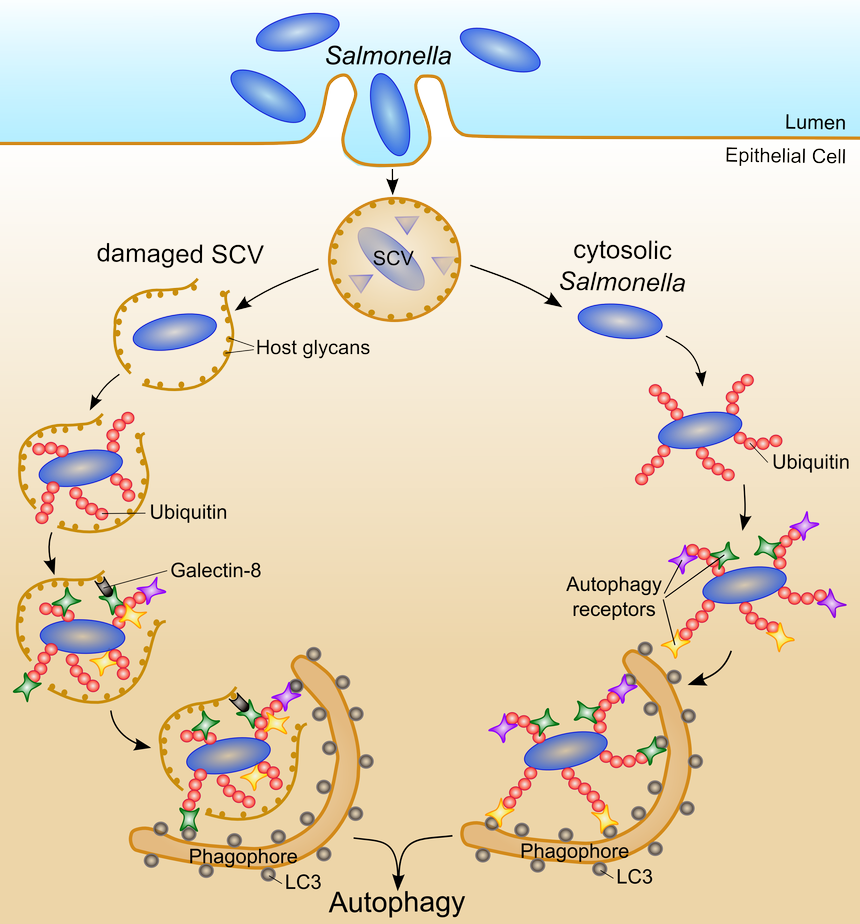
Figure 1: Model of Salmonella ubiquitination and capturing by autophagy. Inside the host Salmonella invades epithelial cells and resides within a specialized intracellular compartment, the Salmonella containing vacuole (SCV). A fraction of these bacteria does not persist inside the SCV, enters the host cytosol, and starts to hyper-replicate. Cytosolic Salmonella can be still inside the damaged SCV (left part) or can lose its membrane remains and get totally free in the cytosol (right part). To protect the host cell from hyper-replication, Salmonella is targeted with ubiquitin-chains for the autophagy pathway. The autophagy receptors p62, NDP52, and OPTN serve as adaptors which can bind both ubiquitin-chains and LC3 molecules on phagophores. Additionally, galectin-8 can bind to host glycans exposed on damaged SCVs, and targets Salmonellainside damaged SCVs for autophagic degradation.
Project members
This work is supported by the LOEWE project Ub-Net of the State of Hesse (Germany).
In our group, Molecular Bioinformatics, Ina Koch, Leonie Amstein and Jennifer Scheidel work on the project.
Posters:
Mathematical Model of antibacterial Autophagy. German Conference on Bioinformatics 2014, Bielefeld
Mathematical modeling to explore the role of Ub-receptors in the clearance of intracellular pathogens
Autophagy is a fundamental defense mechanism against invading pathogens. Pathogens, like Salmonella, that invade the host cytosol are targeted with ubiquitin for the autophagic pathway. It is very important to understand this process to find new therapies for invading pathogens, which more and more frequently become resistant to antibiotics.
To gain a better understanding of the biological system of antibacterial autophagy, we use systems biology approaches. The goal of the project is to develop a first mathematical model of ubiquitin dependent host-pathogen interactions. We apply the Petri net formalism to model the processes of Salmonella ubiquitination and the recognition of the autophagy receptors. Beside this, we are interested in the influence of inflammation pathways and the NF-?B pathway induced by Salmonella infection to compare the processes of the LPS-induced inflammation and the sterile inflammation. We apply the tool MonaLisa for the construction, the analysis, and the simulation of the Petri net model.

Figure 1: Model of Salmonella ubiquitination and capturing by autophagy. Inside the host Salmonella invades epithelial cells and resides within a specialized intracellular compartment, the Salmonella containing vacuole (SCV). A fraction of these bacteria does not persist inside the SCV, enters the host cytosol, and starts to hyper-replicate. Cytosolic Salmonella can be still inside the damaged SCV (left part) or can lose its membrane remains and get totally free in the cytosol (right part). To protect the host cell from hyper-replication, Salmonella is targeted with ubiquitin-chains for the autophagy pathway. The autophagy receptors p62, NDP52, and OPTN serve as adaptors which can bind both ubiquitin-chains and LC3 molecules on phagophores. Additionally, galectin-8 can bind to host glycans exposed on damaged SCVs, and targets Salmonellainside damaged SCVs for autophagic degradation.
Project members
This work is supported by the LOEWE project Ub-Net of the State of Hesse (Germany).
In our group, Molecular Bioinformatics, Ina Koch, Leonie Amstein and Jennifer Scheidel work on the project.
Posters:
Mathematical Model of antibacterial Autophagy. German Conference on Bioinformatics 2014, Bielefeld




